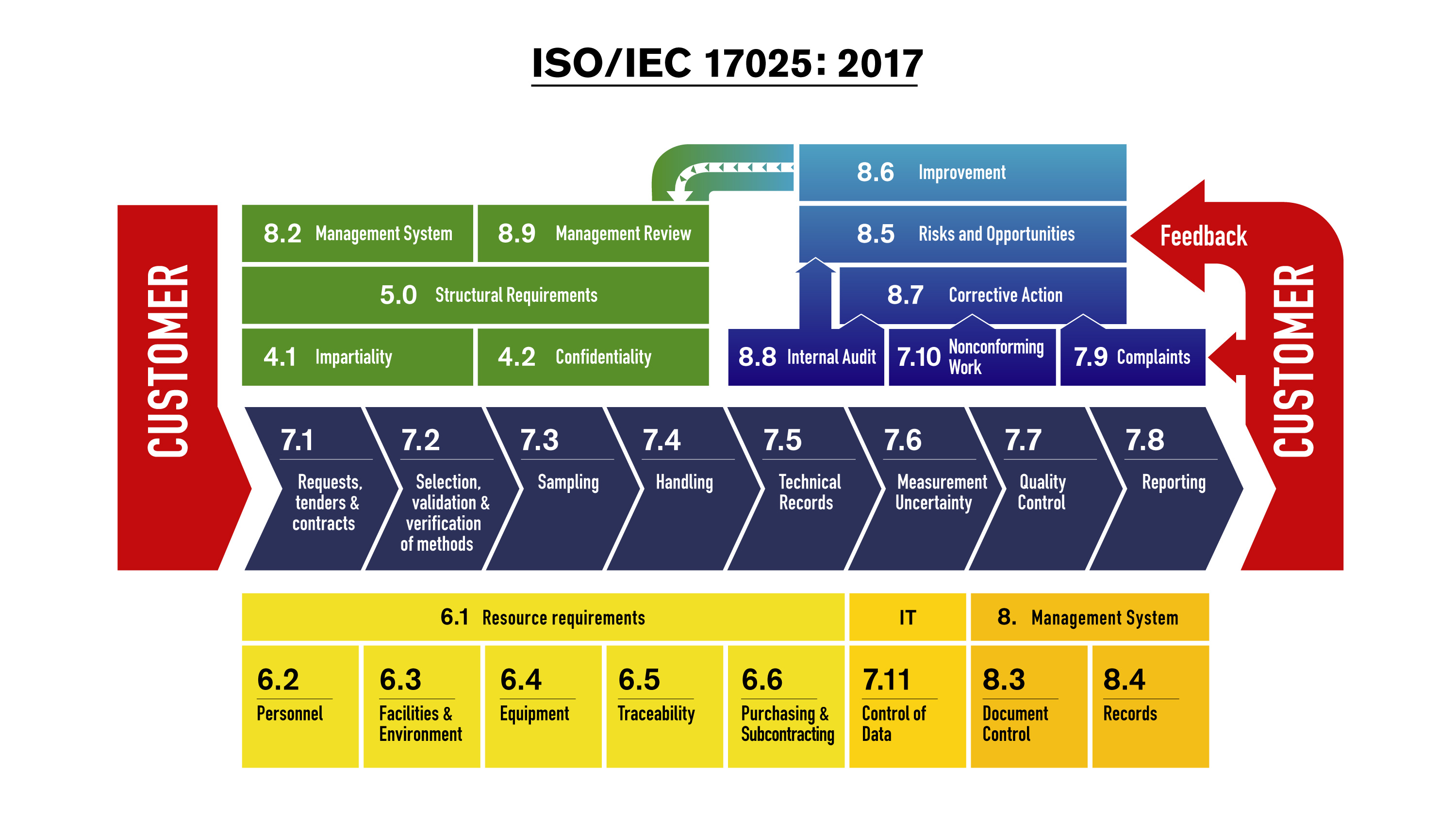
Real-Time RT-PCR Panel for Detection 2019-Novel Coronavirus
Centers for Disease Control and Prevention, Respiratory Viruses Branch, Division of Viral Diseases
Instructions for Use
Printer friendly version pdf icon[PDF]
Purpose: This document describes the use of real-time RT PCR (rT-PCR) assays for the in vitro qualitative detection of 2019-Novel Coronavirus (2019-nCoV) in respiratory specimens and sera. The 2019-nCoV primer and probe sets are designed for the universal detection of SARS-like coronaviruses and for specific detection of 2019-nCoV.
Protocol Use Limitations: The rRT-PCR assays described here have not been validated for platforms or chemistries other than those described in this document.